Piano-stool N-heterocyclic carbene iron complexes and the impact of ligand variation on hydrosilylation activity
Hydrosilylation of alkenes and carbonyls is an important catalytic transformation both in bulk and fine chemical industry, however this transformation is normally catalyzed by precious metals such as platinum rhodium and iridium.1,2 In the past two decades iron catalysts have emerged as attractive complexes for this transformation, offering a cheaper and more benign option. While NN and NNN type ligands dominate the hydrosilylation of alkenes, N-heterocyclic carbenes (NHCs) have demonstrated efficiency as ligands for iron-catalyzed hydrosilylation of carbonyls.3,4,5
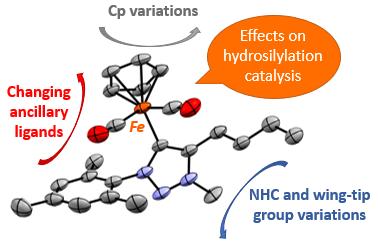
In this work we present a new synthetic method to prepare NHC iron piano-stool complexes and we evaluate effects emerging from variations of wing-tip groups, NHC scaffold, cyclopentadiene (Cp) unit and ancillary ligands on catalytic hydrosilylation activity. Our data reveal a clear trend in catalytic activity which depends strongly on the NHC scaffold as well as the steric bulk imparted by the NHC wing-tip groups. We will also discuss mechanistic insights obtained by 1H NMR and in situ spectroscopies, which support a substrate-dependent catalyst activation.6
[1] Robin Hofmann, Matea Vlatkovic, Frank Wiesbrock, Polymers, 2017, 9, 534.
[2] Álvaro Raya-Báron, Pascual Oña-Burgos, Ignacio Fernández, ACS Catalysis, 2019, 9, 5400-5417
[3] Suzanne Bart, Emil Lobkovsky, Paul Chirik, J. Am. Chem. Soc. 2004, 126, 13794−13807.
[4] Jessica Wu, Benjamin Stanzl, Tobias Ritter, J. Am. Chem. Soc. 2010, 132, 13214−139216
[5] Chloë Johnson, Martin Albrecht, Organometallics, 2017, 36, 2902-2913
[6] Pamela Nylund, Martin Albrecht, Organometallics, 2021, 40, 1538-1550