A Biomimetic Superreduced Iron-Sulfur Cubane and its Oxidized Analogues
In nature, cystein-ligated iron-sulfur (FeS) cubanes of Ferredoxins (Fds) and High-Potential Iron-Sulfur Proteins (HiPIPs) mediate electron transfer and are known to exist in four different oxidation states, namely [Fe4S4]0/1+/2+/3+.[1,2] Among these, the elusive “superreduced” all-ferrous state, [Fe4S4]0, is only retained by the Iron Protein (FeP) of the Nitrogenase enzyme, which belongs to the Fd family.[3–5] Pioneered by R. Holm in the early 70s, bioinorganic chemists have isolated and characterized a variety of FeS cubanes. However, a systematically comparable redox series, which bears the same thiolate ligands and counter-cations and covers all biologically relevant oxidation states, including [Fe4S4]0, is yet lacking.[6–8]
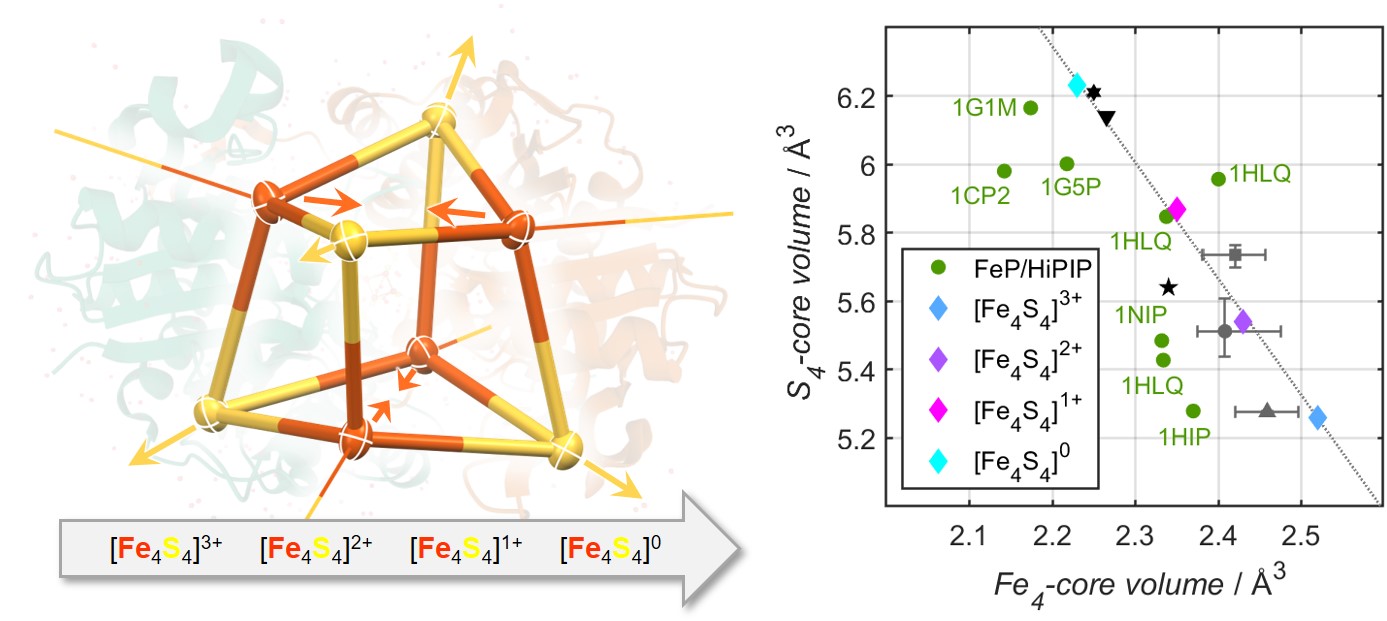
Herein we report the synthesis of [Fe4S4(SR)4]4- via twofold chemical reduction of a novel cubane, [Fe4S4(SR)4]2-, in its traditional oxidation state. To the best of our knowledge, [Fe4S4(SR)4]4- is the first example of an all-ferrous FeS cubane supported by thiolate ligands, that is isolable in substance and characterized by X-ray diffraction analysis. Its oxidation state assignment is supported by extensive spectroscopic characterization including UV-Vis, EPR, XAS, EXAFS as well as variable-temperature 57Fe Mössbauer spectroscopy. Further, for the first time, using straightforward redox-chemistry, all biologically pertinent oxidation states of this new FeS cubane system, [Fe4S4(SR)4]n (n = 1-, 2-, 3-, 4-), are accessible. X-ray diffraction analysis of all members of this biomimetic electron-transfer series reveals that reduction of the Fe4S4-core is accompanied by structural compression of the Fe4-tetrahedron and a concomitant expansion of the S4-tetrahedron in a linear relationship. Thereby, because the S4-expansion supersedes the Fe4-contraction, the overall FeS cubane-volume as well as its surface area are being maximized, which confirms the long-standing hypotheses of biological studies.[5]
[1] S. C. Lee, W. Lo, R. H. Holm, Chem. Rev. 2014, 114, 3579–3600. [2] H. L. Rutledge, F. A. Tezcan, Chem. Rev. 2020, 120, 5158–5193. [3] G. D. Watt, K. R. N. Reddy, J. Inorg. Biochem. 1994, 53, 281–294. [4] H. C. Angove, S. J. Yoo, B. K. Burgess, E. Münck, J. Am. Chem. Soc. 1997, 119, 8730–8731. [5] P. Strop et al., Biochemistry 2001, 40, 651–656. [6] T. A. Scott, C. P. Berlinguette, R. H. Holm, H.-C. Zhou, Proc. Natl. Acad. Sci. 2005, 102, 9741–9744. [7] L. Deng, R. H. Holm, J. Am. Chem. Soc. 2008, 130, 9878–9886. [8] E. Münck, E. L. Bominaar, Science 2008, 321, 1452–1453.