Fe(TMP)2 - Synthesis, characterisation and tuneable reactivity of a new Fe(II) bis(amide) complex
Two coordinate first-row transition metal complexes are rare but key class of reagent for the synthetic inorganic chemist. Such coordinatively unsaturated open-shell complexes are capable of displaying unique reactivity with small molecules, interesting coordination chemistry and often possess stimulating magnetic properties.[1] Since the introduction of the HMDS amido ligand (HMDS = hexamethyldisilazide) in the 1960's, MII(HMDS)2 complexes such as Fe(HMDS)2, originally reported by Lappert and co-workers in 1988,[2] have been prepared and utilised to great extent as key synthetic precursors owing to their ease of synthetic access, high solubility and ligand lability. Recently we have utilised Fe(HMDS)2, in combination with sodium amides, for the regioselective deprotonation and ferration of non-activated arenes.[3]
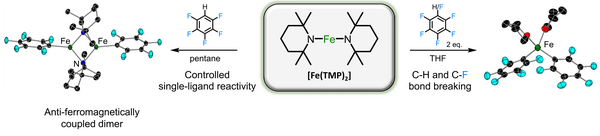
Breaking new ground in this field, here we introduce Fe(TMP)2 as a new addition to the class of two-coordinate first-row transition metal complexes. Possessing highly basic TMP (TMP = tetramethylpiperidide) amido ligands, this iron bis(amide) exhibits unprecedented reactivity, capable of breaking C-H and strong C-F bonds, without the presence of a strong alkali-metal base. The synthetic routes to access this novel iron amide and its inherent properties, including structural, spectroscopic and magnetic characterisation will also be discussed. Furthermore, we detail its novel and tuneable reactivity towards fluoroarenes, providing mechanistic insight through DFT calculations.
[1] P. P. Power, Chem. Rev. 2012, 112, 3482–3507.
[2] R. A. Andersen, K. Faegri, J. C. Green, A. Haaland, M. F. Lappert, W. P. Leung, K. Rypdal, Inorg. Chem. 1988, 27, 1782–1786.
[3] L. C. H. Maddock, M. Mu, M. Garcia-Melchor, E. Hevia, Angew. Chem. Int. Ed. 2021, anie.202104275.