AmoMax®-Casale: from the concept to the implementation in industrial reactors
Catalytic ammonia synthesis from H2 and N2 represents one of the most important industrial reactions today. The catalyst used in this reaction is made from iron oxide with small amounts of other oxides added as promoters to enhance activity and stability. Despite the Haber-Bosch process being more than 100 years old [1-3], only incremental improvements have been achieved until recently. Combining the catalyst expertise of CLARIANT and the engineering knowledge of CASALE, a breakthrough has been realized leading to the new ammonia synthesis catalyst AmoMax®-Casale. The catalyst is a customized design by CLARIANT for CASALE reactors (patent pending) with significantly improved activity compared to state-of-the-art iron-based catalysts. When introducing a new catalyst into the market, performance evaluation is of utmost importance, but simple tests of the catalyst in powder form are not representative enough for industrial applications and only suitable for screening purposes. Therefore, a precise and rigorous methodology must be applied. The AmoMax®-Casale was awarded with the ICIS Best Sustainable process 2020 [4] and with the Sandmeyer Award 2021 [5].
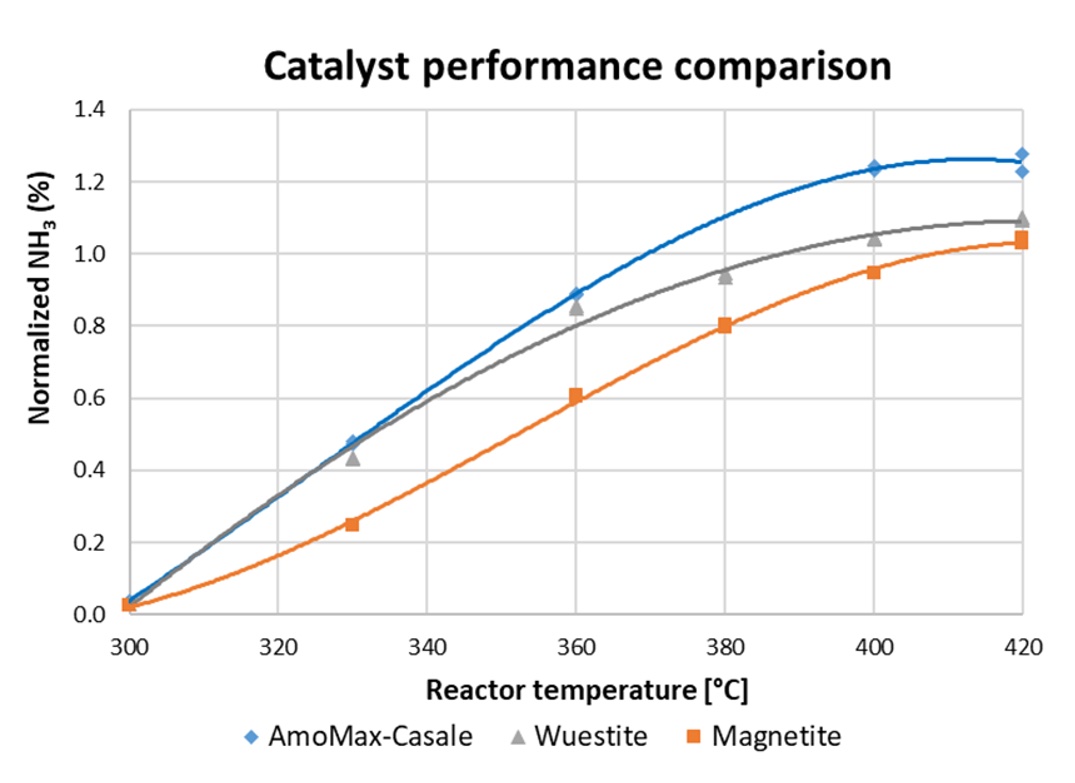
Figure 1. Comparison between state-of-the art magnetite-based catalyst, wuestite based catalyst and AmoMax®-Casale
[1] Huazhang Liu, Ammonia Synthesis Catalysts Innovation and practice. Chemical Industry Press (2013).
[2] Martyn V. Twigg, Catalyst Handbook, Second Edition. Wolf Publishing Ltd. (1989).
[3] Robert Jennings, Catalytic Ammonia Synthesis Fundamentals and Practice. Springer (1991).
[4] https://eu.eventscloud.com/ehome/innovationawards/Home/ consulted on the 19.01.2021
[5] https://scg.ch/index.php?option=com_content&view=category&layout=blog&id=91&Itemid=590&lang=en consulted on the 19.01.2021