Mn(I) Phosphine-Amino-Phosphinites: A Highly Modular Class of Pincer Complexes for Enantioselective Transfer Hydrogenation of ketones
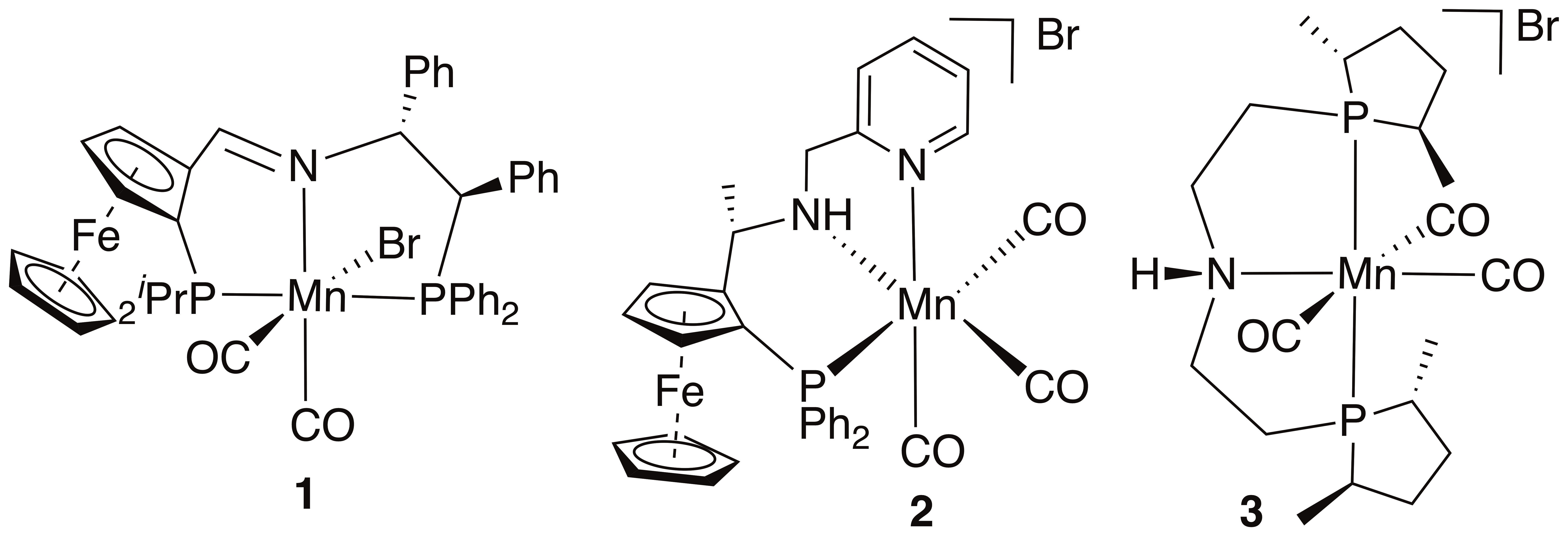
Despite manganese being cheap and non-toxic, the first Mn(I) catalysts for the asymmetric hydrogenation of ketones were reported only in 2017. Complex 1 is active under hydrogen transfer conditions (ATH), whereas 2 and 3 operate under H2 (AH),1 and all contains pincer ligands.
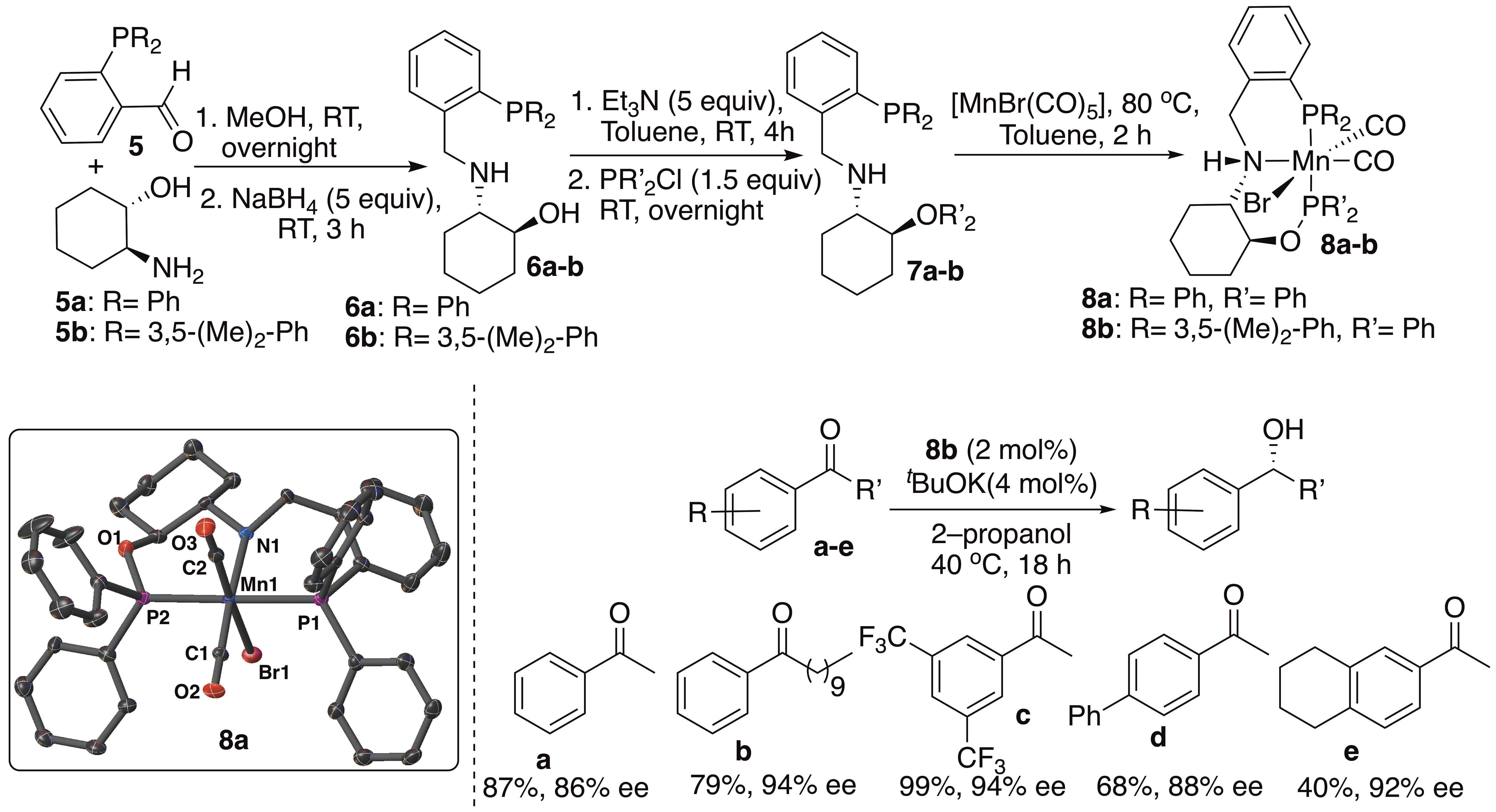
We report here, a new class of chiral phosphine-amino-phosphinite pincer ligands (P’(O)N(H)P) (7a-b) and their Mn(I) complexes (8a-b) that are prepared from cheap (1S,2S)-2-aminocyclohexanol. Complexes 8a-b are active only under ATH conditions. Catalyst 8b (2 mol%, tBuOK (4 mol%), 2-propanol, 40 °C, 18h) hydrogenates aromatic ketones to their corresponding alcohols with enantiomeric excess and conversion up to 97% ee and 99%, respectively (selected examples given below).
[1] (a) Afrooz Zirakzadeh, Sara R. M. M. de Aguiar, Berthold Stöger, Michael Widhalm, Karl Kirchner, ChemCatChem 2017, 9, 1744-1748. b) Magnus B. Widegren, Gavin J. Harkness, Alexandra M. Z. Slawin, David B. Cordes, Matthew L. Clarke, Angew. Chem. Int. Ed.2017, 56, 5925-5828. c) Marcel Garbe, Kathrin Junge, Svenja Walker, Zhihong Wei, Haijun Jiao, Anke Spannenberg, Stephan Bachmann, Michelangelo Scalone, Matthias Beller, Angew. Chem. Int. Ed. 2017, 56