Suppression of water inhibition and thermal deactivation on Pd/Al2O3 during lean methane oxidation using oxygen dithering
The growing interest in natural gas as transportation fuel arises from its lower CO2 and particulate matter emissions together with its higher energy density. However, unburnt CH4 is a severe greenhouse gas and has to be removed from the exhaust. Because this is challenging[1], important efforts must be undertaken to improve CH4 abatement either through material development or by optimization of the operating conditions. Previous studies[2,3] have demonstrated that by repeatedly applying short reducing pulses (SRP), which are implemented by periodic removal of O2 from the gas feed, a significant increase in activity could be achieved for lean CH4 oxidation and NG exhaust treatment over a Pd-based zeolite[2] and a three-way catalyst[3], respectively.
In this study[4], the beneficial effect induced by short reducing pulses were studied over a Pd/Al2O3 catalyst for wet lean CH4 oxidation. By applying SRP, we were able to activate the catalyst and bring it to a highly active state compared to static conditions (T50% from 428 °C to 370 °C; Figure 1a). In line with this observation, the EA,app under SRP conditions dropped by ca. 20 kJ/mol. This strategy also allowed reversing the structural effects induced by thermal treatments and therefore to recover the degraded activity of these materials. Consequently, under isothermal conditions (435 °C), SRP suppressed the fast deactivation of Pd/Al2O3 (fresh and aged), observed under static conditions in the presence of water, and allowed to maintain the catalyst active (> 99%) for over 16 h (Figure 1b). Combination of time-resolved operando X-ray absorption spectroscopy (XAS) at the Pd K-edge and fit of oxidation kinetic models revealed that only moderately active PdO species were present under static conditions while under SRP operation an amorphous PdOx shell around a Pd metallic core was formed and maintained for long periods (Figure 1c). This study demonstrates that high activity can be achieved in presence of water as long as a Pd metallic core is present, but when this is fully consumed, PdOx is believed to densify and crystallize causing a decrease of CH4 conversion. Based on these results we propose a practical range of Pd oxidation degree that allow maintaining high CH4 conversion levels.
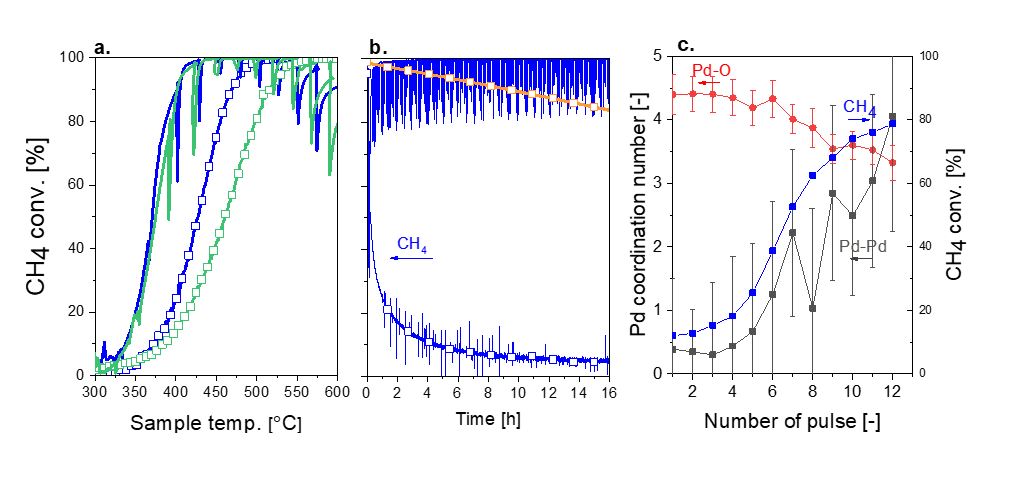
Figure 1. CH4 conversion of fresh catalyst (blue) and thermally aged catalyst (green) (a) during heating ramp and (b) stability test under static (-□-) and SRP (-) lean conditions. Panel b. includes data collected for the fresh sample in dry static conditions (green). Conditions: 1 vol% CH4, 4 vol% O2, 10 vol% H2O; WHSV = 2,550 h-1 mg-1.
[1] R. W. Howarth, Energy Sci. Eng. 2014, 2, 47–60.
[2] A. W. Petrov, D. Ferri, F. Krumeich, M. Nachtegaal, J. A. Van Bokhoven, O. Kröcher, Nat. Commun. 2018, 9, 1–8.
[3] D. Ferri, M. Elsener, O. Kröcher, Appl. Catal. B Environ. 2018, 220, 67–77.
[4] T. Franken, M. Roger, A. W. Petrov, A. H. Clark, M. Agote-Arán, F. Krumeich, O. Kröcher, D. Ferri, ACS Catal. 2021, 11, 4870–4879.