Diformylxylose as a New Polar Aprotic Solvent Produced from Renewable Biomass
The gradual depletion of fossil resources, increase in global energy consumption, and the environmental issues encourage the development of new chemicals and materials produced from renewable sources. The development of bio-based solvents that could compete with petroleum-derived analogs is an area of high interest to chemical and pharmaceutical industry. Recently, ACS Green Chemistry Institute® Pharmaceutical Roundtable identified the development of viable replacements for polar aprotic solvents (PAS) as a key green chemistry research area.1 PAS possess unique characteristics such as high polarity and low reactivity, which makes them excellent media for the production of active pharmaceutical ingredients. However, many commonly used PAS are extremely hazardous, mutagenic, and negatively impact the environment, which provokes regulatory response. For example, European Union REACH has restricted the industrial use of N-methylpyrrolidinone (NMP), N,N-dimethylacetamide (DMAc) and N,N-dimethylformamide (DMF) due to their severe reproductive toxicity. Therefore, increasing regulatory and commercial pressure drives switching to greener alternatives such as biomass-derived GVL, 2-Me-THF, Cyrene and other emerging solvents. However, current replacement candidates do not meet all safety requirements, often lack necessary solvation and physical properties, and still considered as “problematic” according to industrial solvent selection guides. Another big challenge is the high production cost of bio-based PAS because aprotic molecules are rarely found in abundance in the natural world and their production from protic ones (e.g., carbohydrates, carboxylic acids, lignin, alcohols, etc.) often requires multi-step processes, sometimes involving metals and high pressure. This factor can be strongly reduced in the case of acetal-stabilized xylose – namely, Diformylxylose (DFX), that can be produced from biomass in almost 100% yield (on xylan basis)2 or directly from xylose3 using inexpensive chemicals and common equipment. In this work, we are introducing Diformylxylose in the role of bio-based solvent with polar aprotic nature.
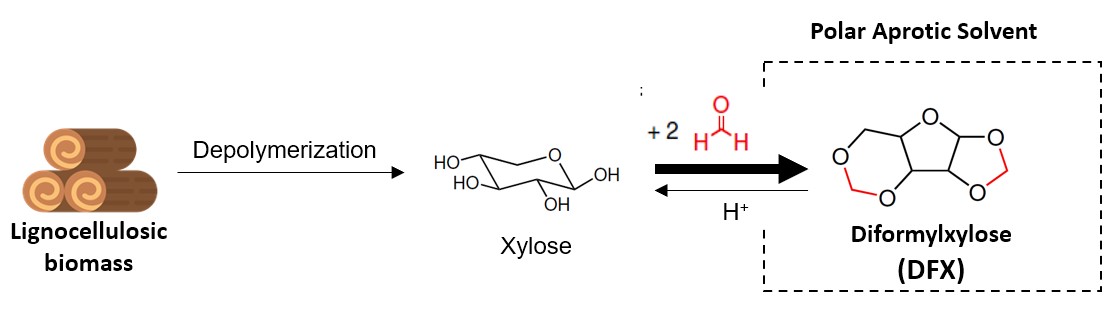
Diformylxylose demonstrated similar performance to conventional PAS such as DMF, NMP, DMSO in alkylation, cross-coupling, and hydrogenation reactions, while having a safer profile. Solvation properties of DFX were fully characterized by two models - Kamlet-Abboud-Taft solvatochromic parameters and Hansen Solubility Parameters. We demonstrated that DFX possesses unique solvation properties — high polarity and high hydrogen-bond accepting ability. Physical properties of DFX such as high boiling (237°C) and melting point (48°C) indicate a lower risk of human exposure and the environmental impact due to low volatility. Finally, toxicological assessment (bacterial Ames test) showed that DFX is a non-mutagenic and non-carcinogenic molecule. Overall, low production cost, high performance, non-mutagenic nature, and renewability make DFX a promising bio-based alternative to traditional polar aprotic solvents.
[1] M. C. Bryan, P. J. Dunn, D. Entwistle, et al, Green Chem., 2018, 20, 5082–5103.
[2] M. Talebi Amiri, G. R. Dick, Y. M. Questell-Santiago and J. S. Luterbacher. Nat. Protoc., 2019, 14, 921–954.
[3] Y. M. Questell-Santiago, R. Zambrano-Varela, M. Talebi Amiri and J. S. Luterbacher, Nat. Chem., 2018, 10, 1222–1228.