Ethynylbenziodoxolones: Photocatalysis and Direct Excitation for Difunctionalisation and Deoxyalkynylation
Alkynes are an important functional group for synthetic chemists but also present applications in biochemistry, medicinal chemistry and materials science. Broadening the scope of alkyne synthesis is of great importance. Ethynylbenziodoxolones (EBXs) when combined with photocatalysis have proven themselves to be efficient radical traps.[1] Our first strategy combines the use of photocatalysis to generate selectively enamide and enol-ether radical cationic intermediates that can then be trapped by the EBXs exploiting both the nucleophilic carboxylate and the somophilic alkyne moiety of these reagents.[2] This strategy overcomes the issues associated to the generation of highly reactive electrophilic radicals that has been used to date for radical enamide difunctionalisation. In our second strategy, we aimed to develop a redox neutral approach for deoxyalkynylation of tertiary alcohols using cesium salts. Interestingly, we discovered that not only could this reaction be developed using photocatalysis, it also proceeded through the direct excitation of the EBXs alleviating the need for a photocatalyst altogether.[3] This strategy allows the simple access to all-carbon quaternary alkynes under mild conditions.
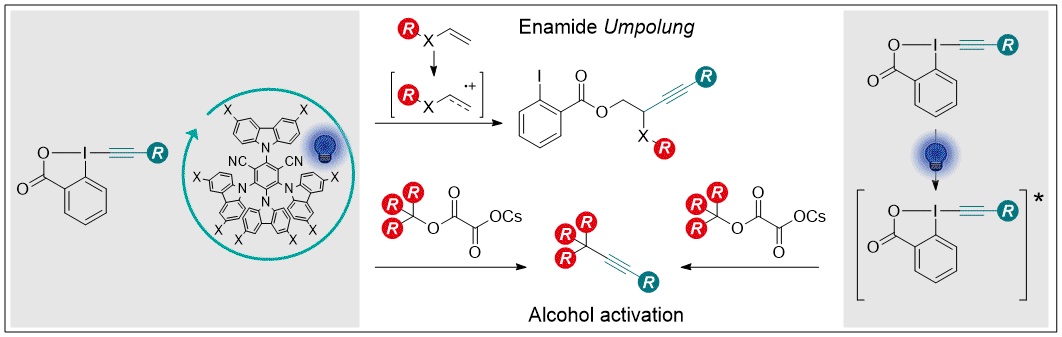
[1] Le Vaillant, F.; Waser, J. Chem. Sci. 2019, 10, 8909–8923.
[2] Amos, S. G. E.; Nicolai, S.; Waser, J. Chem. Sci. 2020, 11, 11274–11279.
[3] Manuscript submitted.