Functionalization of zigzag graphene fragments: Tales of the unexpected
The synthesis of new zigzag nanographenes is key to understand the properties of semiconducting and magnetic materials based on graphene for applications in molecular electronics. The practical issues associated with nanographene compounds are low stability and low solubility, which make routine processability and characterization highly challenging. These obstacles can be overcome by the installment of substituents around the periphery. Nucleophilic addition of carbon-centered nucleophiles to nanographene ketones represents a convenient late-stage method for the peripheral functionalization that can solve the aforementioned problems. The use of this method, however, is rare in the chemical literature, which is surprising considering the vast number of nanographene ketones that have been reported up to now. We reasoned that the scarcity of this method is due to the lack of understanding of the rules that govern it. We therefore explored the nucleophilic addition in more detail, using two topologically different zigzag model systems, non-Kekulé triangulenedione (left) and Kekulé anthanthrone (right). Over the course of this exciting study, we identified unexpected regioselectivities, which we could all rationalize as an interplay of the electronic effects, expressed by the Fukui functions, and sterics related to the size of nanographene and nucleophile.[1] We compiled these findings into a set of rules, which can be used to not only explain the few known examples with unexpected selectivity, but also to predict the selectivity for unknown compounds. As a result, this method can now be used to prepare new nanographene derivatives, which would otherwise only be accessible via long multistep synthesis. This presentation will also include our latest unpublished results, where we utilized these rules to construct stable derivatives of highly reactive longer acenes.
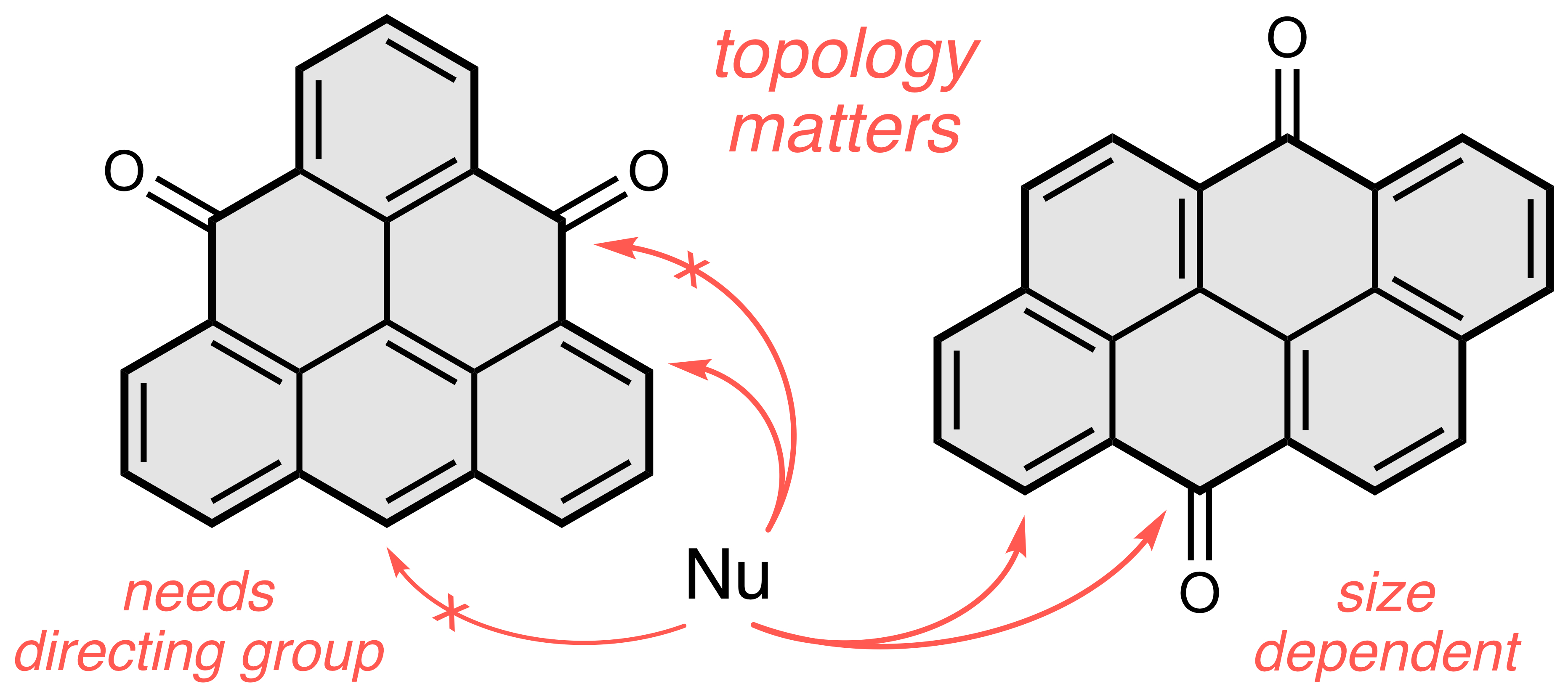
Peter Ribar, Leoš Valenta, Tomáš Šolomek, Michal Juríček, Angew. Chem. Int. Ed., 2021, DOI: 10.1002/ange.202016437