Möbius Carbon Nanohoops
Prediction [1] and synthesis [2] of annulenes twisted into a Möbius strip introduced a new perspective on aromaticity, one of the most important topics in organic chemistry. The conceptually simplest approach to access a molecular Möbius strip is a combination of building blocks with linear and radial π-conjugation.[3] While the former are ubiquitous in π-conjugated systems, the latter require pyramidalization of sp2-hybridized carbons, consequently introducing strain, which hampers the synthesis of these molecules.
One of the recent examples of radially conjugated π-systems are cycloparaphenylenes which possess a considerable strain energy. Preparation of such strained systems is challenging and usually utilizes aromatization as the driving force to overcome the energy penalty. Moreover, cycloparaphenylenes display size-dependent optoelectronic properties that are the direct consequence of radial π-electron conjugation. [4]
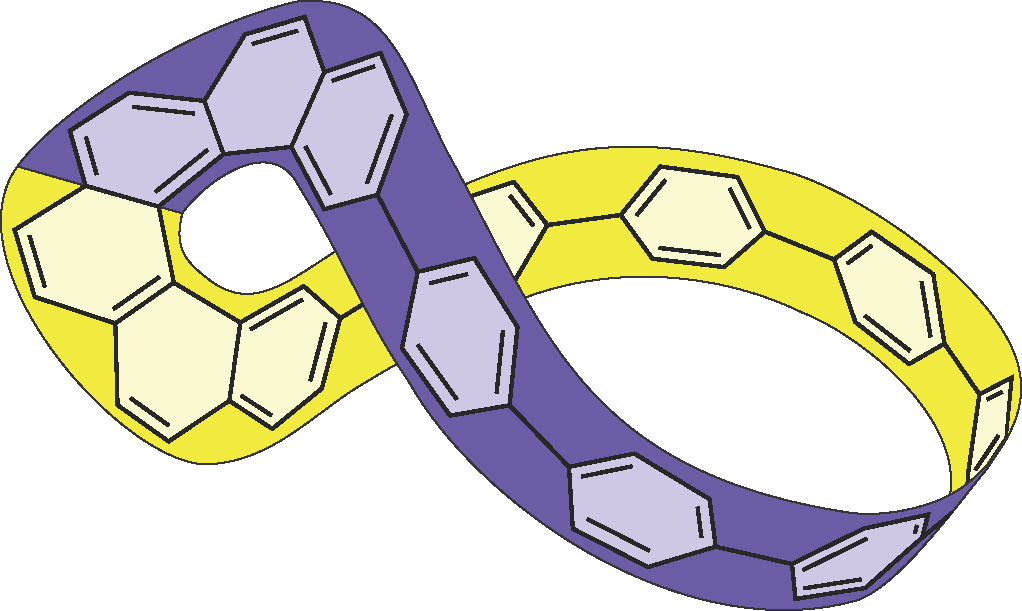
In this work, we incorporated [6]helicene into [7]cycloparaphenylene system creating a chiral helicene carbon nanohoop (Figure 1). [5] The helicene provides the necessary twist that transforms the radial π-conjugation of cycloparaphenylenes into the Möbius topology. We separated the enantiomers of the synthesized helicene carbon nanohoop using HPLC with a chiral stationary phase and demonstrated that its optoeletronic and chiroptical properties arise from the cycloparaphenylene and the helicene moieties, respectively. We investigated the dynamics of the synthesized nanohoop in solution by variable temperature 1H NMR and DFT calculations . Furthermore, we studied the effect of the Möbius topology of the helicene carbon nanohoop and its derivatives on their global aromaticity.
[1] E. Heilbronner, Tetrahedron Lett., 1964, 5, 1923–1928.
[2] D. Ajami, O. Oeckler, A. Simon and R. Herges, Nature, 2003, 426, 819-821.
[3] R. Herges, Chem. Rev., 2006, 106, 4820-4842.
[4] E. R. Darzi, R. Jasti, Chem. Soc. Rev., 2015, 44, 6401–6410.
[5] J. Malinčík, S. Gaikwad, M. Boillat, D. Häussinger and T. Šolomek, ChemRxiv, 2021, Preprint. https://doi.org/10.26434/chemrxiv.13817498.v1