Iron and Cobalt Nitrilimide Complexes: Transformations to Nitride and Isocyanoamide Complexes via a Fleeting Diazomethanediide Intermediate.
In a series of works, Rolf Huisgen and co-workers established that the thermal decomposition of tetrazole derivatives yields fleeting nitrilimines (RNNCR’).[1] These intermediates were trapped upon their 1,3-dipolar cycloaddition with various substrates in a so-called Huisgen reaction. Guy Bertrand and co-workers isolated nitrilimines stabilized with bulky electron-withdrawing substituents at both C and N ends.[2] Building on the latter discovery, we envisaged that the redox of transition metals and the high reactivity of nitrilimines, brought together in transition metal nitrilimide complexes, potentially could give access to unique reactivity.
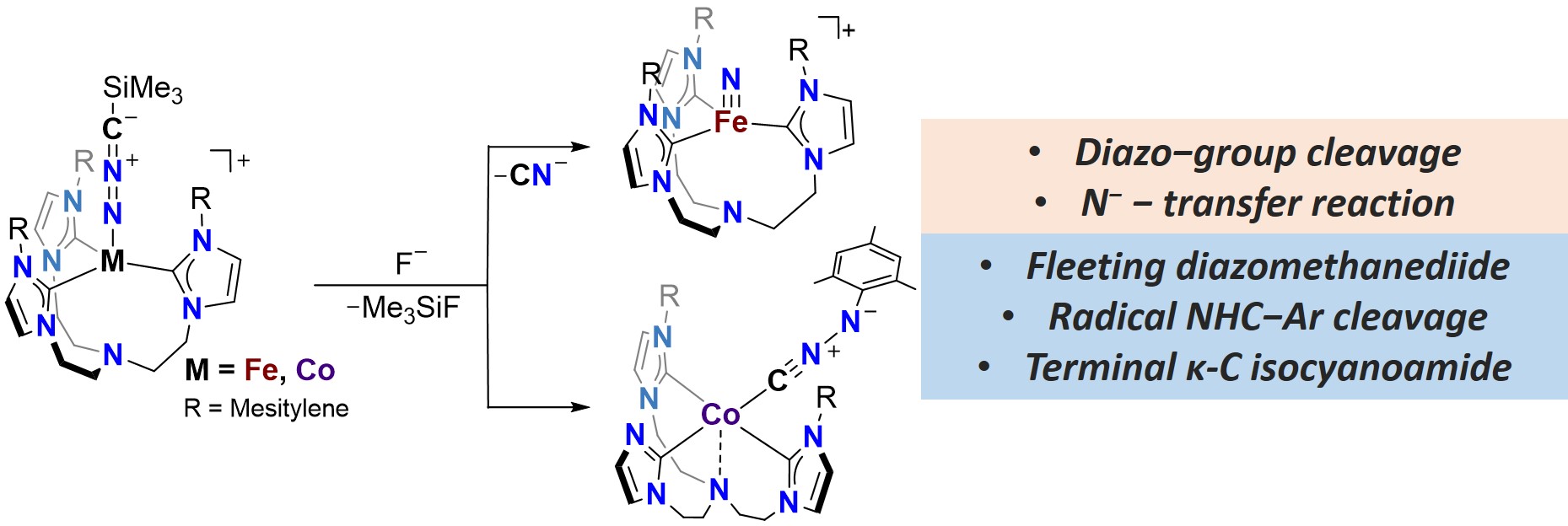
In this contribution, we will present a novel strategy to access transition metal nitrilimide complexes. Specifically, we have isolated Fe(II), and Co(II) nitrilimide complexes [LM−N=N+=C−−SiMe3] stabilized within a tris-NHC ligand.[3,4] A suite of spectroscopic tools, crystallography analysis, and quantum-mechanical calculations revealed detailed electronic structures of nitrilimide complexes. The iron nitrilimide complex undergoes an unprecedented diazo group cleavage upon trimethylsilylium “SiMe3+’’ group removal, giving entry to an iron nitride complex at mild reaction conditions.[3] Due to the inaccessibility of a cobalt nitride complex within the employed tris-NHC ligand, the cobalt nitrilimide complex engages in an alternative transformation upon “SiMe3+’’ removal, forming a novel Co(II) complex featuring a terminal isocyanoamide ligand.[4] Quantum mechanical study suggests that the cobalt nitrilimide-to-isocyanoamide transformation proceeds via a fleeting intermediate featuring a diazomethanediide ligand. The understanding of the reactivity of nitrilimide complexes could be further implemented in designing alternative systems for unique element or group transfer reactions.
[1] R. Huisgen, M. Seidel, J. Sauer, J. McFarland, G. Wallbillic, J. Org. Chem. 1959, 24, 892−893;
[2] G. Sicard, A. Baceiredo, G. Bertrand, J. Am. Chem. Soc. 1988, 110, 2663−2664;
[3] S. Aghazada, M. Miehlich, J. Messelberger, F. W. Heinemann, D. Munz, K. Meyer, Angew. Chem. Int. Ed. 2019, 58, 1854718551;
[4] S. Aghazada, D. Fehn, F. W. Heinemann, D. Munz, K. Meyer, Angew. Chem. Int. Ed. 2021, 60, 11138−11142.