Exploring the function of microtubule post-translational modifications by semi-synthetic tubulin
Microtubule filaments, critical for cell structure, division and motility, are comprised of α- and β-tubulin dimers containing C-terminal tails with highly variable post-translational modifications (PTMs) such as detyronisation, polyglutamylation and polyglycylation. The tightly regulated spatial and temporal distribution of PTMs in the microtubule network underlines its importance for many cellular processes involving this major cytoskeleton component. Microtubules can form a local, specialized support as they are decorated by combinations of PTMs, which has been aptly described as the ‘microtubule code’.1 However, deciphering the microtubule code to unravel its role in cell biology or pathogenesis remains challenging. A major difficulty in studying PTMs lays in the unavailability of uniformly modified microtubules as we are limited to mostly natural resources of tubulin (i.e. cow or porcine brain cells).
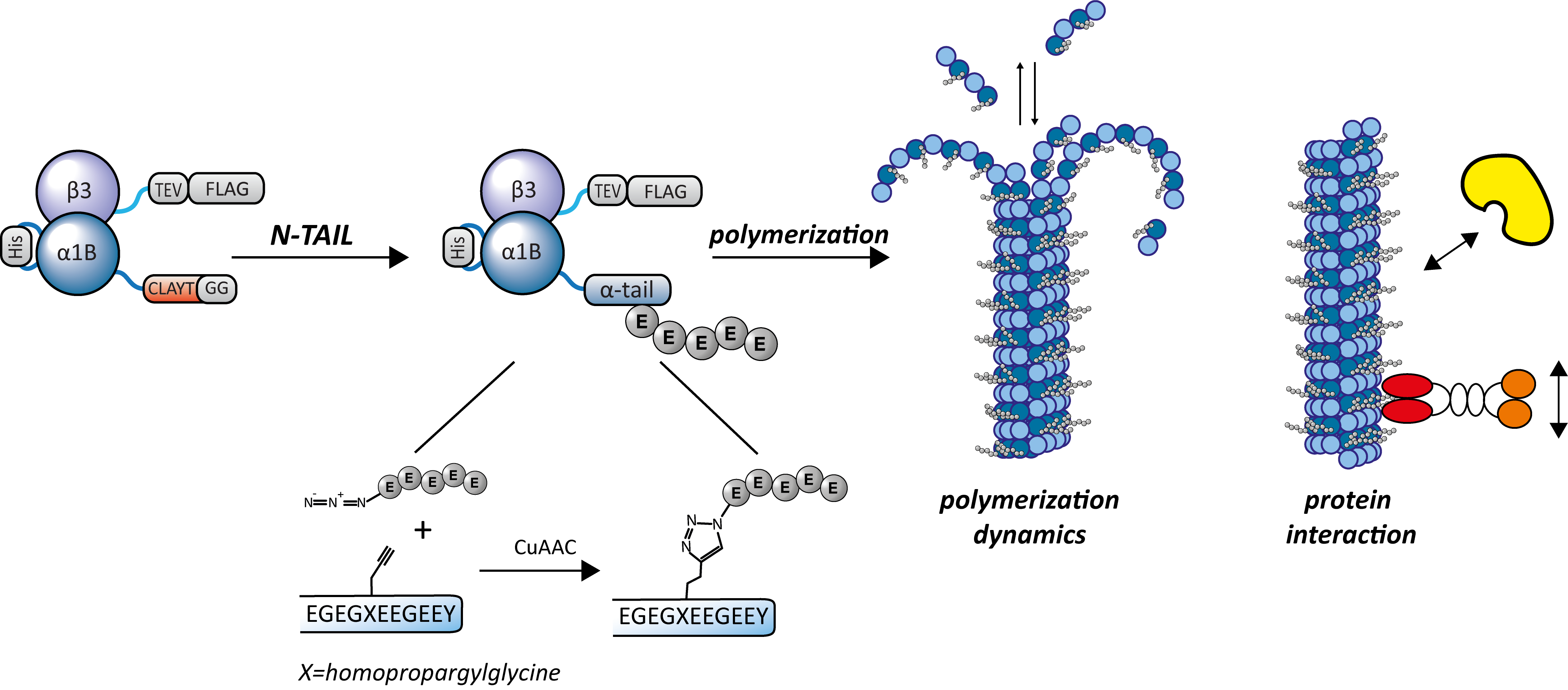
Here we overcome this limitation by developing a semi-synthetic tubulin; a chemically defined C-terminal tail is fused to recombinant purified tubulin dimers. A recently developed ligation method, transpeptidase-assisted intein ligation (TAIL),2 appeared extremely suitable for addition of the tubulin C-terminal tail as it requires a small recognition motif and only leaves a two amino-acid scar. We demonstrate the use of TAIL, where initially 1) a Sortase enzyme ligates an N-terminal intein onto the α-tubulin followed by 2) a protein trans-splicing reaction to fuse a pre-designed synthetic C-terminal tail bearing the intein counterpart. The two intein halfs can reassemble and propagate a thiolester-cysteine mediated ligation, excising itself and generating the semi-synthetic tubulin. With full chemical control of the α1B C-terminal tail composition (e.g. enabling the use of copper-catalyzed azide-alkyne cycloadditions) we produced a small set of α1B-tubulins containing either fluorescein or branches of one, five or ten glutamates. Dimers of α1B/β3 with the different PTMs were assessed for their microtubule polymerization dynamics and interaction with modifiers such as the newly discovered detyronisation enzyme vasohibin.
With this technology we will be able to test the individual effect of defined tubulin PTMs in a highly defined system using semi-synthetic tubulin proteins, carrying specific PTM patterns. These unique reagents will thus provide a key to decipher the tubulin code.
[1] Sudarshan Gadadhar, Satish Bodakuntla, Kathiresan Natarajan, Carsten Janke, Journal of Cell Science, 2017, 130, 1347-1353.
[2] Robert E Thompson, Adam J Stevens, Tom W Muir, Nature Chemistry, 2019, 11, 737-743.