Towards Sustainable Catalysis: Development of Chiral α-Diimine Iron Complexes for Asymmetric Cycloadditions of 1,3-Dienes
The advent of catalysis has had a life-changing impact on modern society. Asymmetric transformations are nowadays in high demand due to the reduced environmental footprint of single enantiomer production. Despite the majority of synthetic catalysts contain noble metals (e.g. palladium and platinum), biological transformations are mostly catalyzed by Earth-abundant metals (EAM), such as iron and nickel. Their higher terrestrial abundance and lower price is the reason for a gradual switch towards a more sustainable metal catalysis.[1] Moreover, the different electronic properties displayed by noble and 3d-metals open the possibility for exploring unusual reactivity and discovering novel transformations.
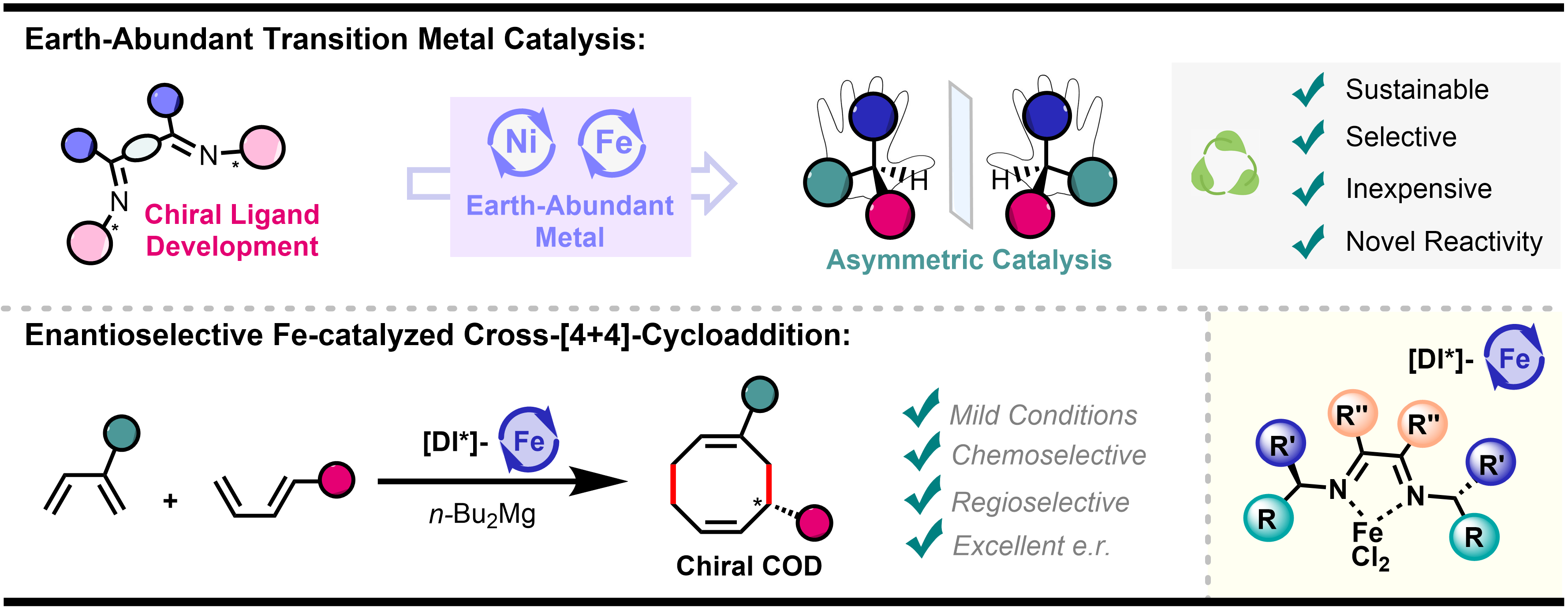
Chiral cyclooctadienes (CODs) are a frequently occurring scaffold in natural products and specialty chemicals, and are used as steering ligands for asymmetric catalysis. They are among the most difficult rings to synthesize, mainly because of unfavorable entropic and transannular penalties, and accessing them in an efficient asymmetric fashion has been a longstanding unsolved issue.
We present the first highly enantioselective iron-catalyzed cross-[4+4]-cycloaddition of 1,3-dienes to form substituted cyclooctadienes in a single step from feedstock materials and under very mild conditions.[2] A highly tailored chiral α-diimine iron complex, easily accessible and tunable, is key for the success of this transformation, providing a balanced performance between reactivity, excellent cross-selectivity and very high enantioselectivity. Analysis of the steric maps of the microenvironment around the iron center helps account for the observed selectivity.
Furthermore, the application of the developed catalyst class in other cycloaddition reactions will be disclosed for the first time.[3]
[1] Bullock et al., Science 2020, 369, 786.
[2] Braconi, E.; Götzinger, A.; Cramer, N. J. Am. Chem. Soc. 2020, 142, 19819. (Highlighted in JACS Spotlights 2020, 142, 19779 and Synfacts 2021, 17, 181)
[3] Work in Progress.