Titanium hydride surfaces for ammonia synthesis
Because of its relevance in catalysis, the interaction of hydrogen with metallic surfaces is generally well studied. However, knowledge on surface properties is limited when it comes to the class of metals, which form bulk hydrides, such as titanium. Hydride surfaces have peculiar properties for their application as heterogeneous hydrogenation catalysts, e.g., they may have even less hydrogen on the surface than their metallic counterparts resulting in lower catalytic yields [1]. Titanium dihydride TiH2 has been recently shown to catalyze the ammonia synthesis under Haber-Bosch conditions while titanium metal shows no activity [2].
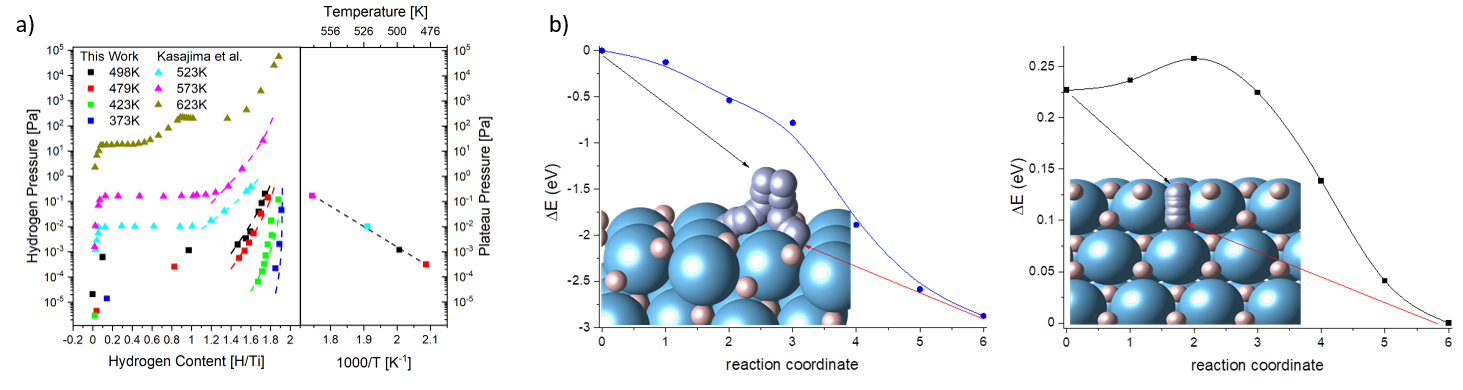
The key to understanding the catalytic properties of hydrides is their electronic structure. The standard electron spectroscopy methods are incompatible with hydrogen pressures needed to form hydrides, therefore these kind of experiments are usually restricted to post-mortem analysis. We have developed a method to hydrogenate thin films in-situ under UHV conditions compatible with electron spectroscopy measurements. We can measure pressure-composition isotherms (pcT) of the Ti-H system by electron energy loss spectroscopy (EELS) (see Fig. a) and investigate different titanium hydrides by electron spectroscopy methods. Furthermore the UHV system allows for introduction of nitrogen gas and the spectroscopic study of the resulting surfaces. The findings are supported by DFT calculations of the titanium hydrogen interactions (see Fig. b).
[1] Emanuel Billeter, Jasmin Terreni, Andreas Borgschulte, ChemPhysChem, 2019, 20, 1398-1403
[2] Yoji Kobayashi, Ya Tang, Toki Kageyama, Hiroki Yamashita, Nayoa Masuda, Saburo Hosokawa, Hiroshi Kageyama, Journal of the American Chemical Society, 2017, 139, 18240-18246
[3] Takeo Kasajima, Tokujiro Nishikori, Toshiyuki Nohira, Yasuhiko Ito, Journal of the Electrochemical Society, 2003, 150, E355