Palladium Catalysed Mild C−H Arylation of Arenes
Transition metal catalysed C–H activation is a transformative methodology for synthesis of complex molecules, allowing new retrosynthetic pathways and late-stage diversification. Non-directed, arene-limited palladium-catalysed C–H functionalisation reactions, such as olefinations, can be accelerated with various neutral and anionic ligands which proceed through CMD mechanism.1,2 However, the direct arylation of C–H bonds without the use of directing groups remains challenging and requires excess of the arene, high temperatures, transition-metal additives, or is limited to certain classes of substrates.3,4
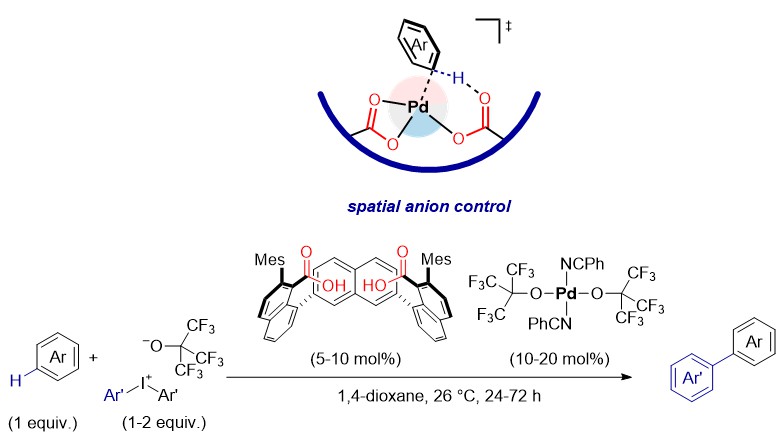
We describe the design of rigid bis(carboxylate) anions which provide spatial anion control on Pd(II), enabling non-directed C–H arylation of arenes at ambient temperature. The catalytic system utilizes palladium(II) precatalysts and iodine(III) arylation reagents with nonafluoro-tert-butoxide anions, which were rationally designed to avoid the use of silver(I) compounds and any other potentially interfering additives or anions.5 The mild conditions enable late-stage structural diversification of biologically relevant small molecules. The calculations show that appropriately spatially positioned carboxylates are powerful cooperative ligands for C–H activation on Pd(II), and could facilitate functionalisation through Pd(IV) intermediates. The concept of spatial anion control offers a general strategy for design of catalytic sites for C–H activation and might find broad use in transition-metal catalysis.
[1] Peng Wang, Pritha Verma, Guoqin Xia, Jun Shi, Jennifer X. Qiao, Shiwei Tao, Peter TW Cheng, Michael A. Poss, Marcus E. Farmer, Kap-Sun Yeung, Jin-Quan Yu, Nature , 2017 , 551 , 480-493.
[2] Hao Chen, Philipp Wedi, Tim Meyer, Dr. Ghazal Tavakoli, Dr. Manuel van Gemmeren, Angew Chem. Int. Ed 2018 , 2497-2501.
[3]Thomas E. Storr, Michael F. Greaney, Org. Lett. 2013, 15, 1410-1413.
[4] Louis-Charles Campeau, Keith Fagnou, Chem. Common. 2006 , 1253-1264.
[5] Jyoti Dhankhar, Elisa González-Fernández, Chao-Chen Dong, Tufan K. Mukhopadhyay, Anthony Linden, Ilija Čorić, J. Am. Chem. Soc. 2020 , 142 , 19040-19046.