TiCl3-Mediated Synthesis of 2,3,3-Trisubstituted Indolenines: Total Synthesis of (+)-1,2-Dehydroaspidospermidine, (+)-Condyfoline, and (−)-Tubifoline
2,3,3-Trisubstituted indolenine constitutes an integral part of many biologically important monoterpene indole alkaloids. We report an unprecedented access to this skeleton by a TiCl3-mediated reductive cyclization of tetrasubstituted alkenes bearing a 2-nitrophenyl substituent.1 The proof of concept is demonstrated firstly by accomplishing a concise total synthesis of (+)-1,2-dehydroaspidospermidine featuring a late-stage application of this key transformation. A sequence of reduction of nitroarene to nitrosoarene followed by 6π-electron-5-atom electrocyclization and a 1,2-alkyl shift of the resulting nitrone intermediate was proposed to account for the reaction outcome.
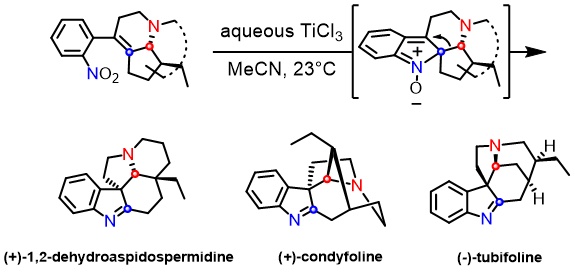
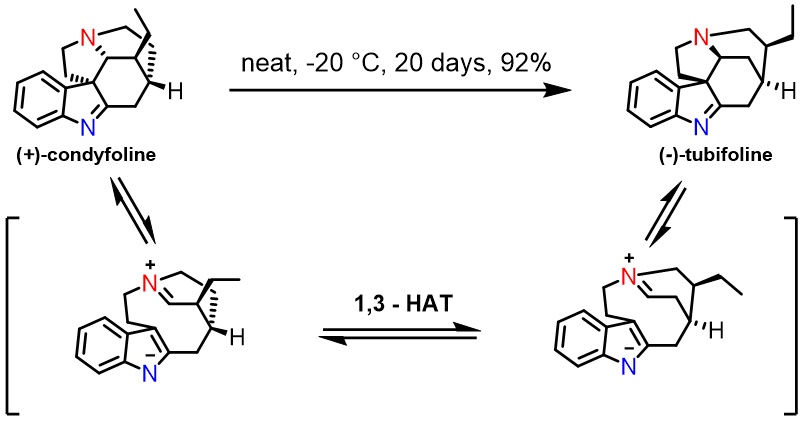
A subsequent total synthesis of (+)-condyfoline not only illustrates the generality of the reaction, but also provides a mechanistic insight into the nature of the 1,2-alkyl shift. The exclusive formation of (+)-condyfoline indicates that the 1,2-alkyl migration follows a concerted Wagner–Meerwein pathway, rather than a stepwise retro-Mannich/Mannich reaction sequence.2 The preservation of the stereochemical integrity of the migrating centres during the rearrangement process is significant, widening the scope of potential targets accessible using this strategy. Conditions for almost quantitative conversion of (+)-condyfoline to (−)-tubifoline by way of a retro-Mannich/1,3-prototropy/transannular cyclization cascade are reported.3 The facile isomerisation of (+)-condyfoline to (-)-tubifoline at low temperature further supports our mechanistic hypothesis.
[1] B. Delayre, C. Piemontesi, Q. Wang, J. Zhu, Angew. Chem. Int. Ed., 2020, 59, 13990-13997
[2] B. Delayre, Q. Wang, J. Zhu, ACS Cent. Sci., 2021, 7, 559-569
[3] D. Schumann, H. Schmid, Helv. Chim. Acta., 1963, 46, 1996-2003