Fully Solvated, Monomeric ReII Complexes: Insights into the Chemistry of [Re(NCCH3)6]2+
The coordination chemistry of the group 7 elements rhenium and technetium is very versatile and takes place across many different oxidation states, usually ranging from +VII to 0. Of course, not all oxidation states are encountered to a similar extent. The most prominent oxidation state is +V, whose d2-system is stabilized by π-donors such as oxo or nitrido. Over the past few years the numbers of complexes with the oxidation state +I increased, especially due to the development of suitable precursor compounds such as the tricarbonyl-cores ({fac-M(CO)3}+, M = Re, 99(m)Tc). A relatively rarely encountered oxidation state is +II. One of the reasons for the small amount of Tc and Re complexes in the oxidation state +II is the lack of stable but also reactive precursor complexes. In general, ReII and TcII compounds are produced by the oxidation of a MI species or by the reduction of a higher valent complex (e.g. MIII). The development of suitable precursor complexes might therefore significantly contribute to the syntheses of novel rhenium and technetium complexes in the oxidation state +II.
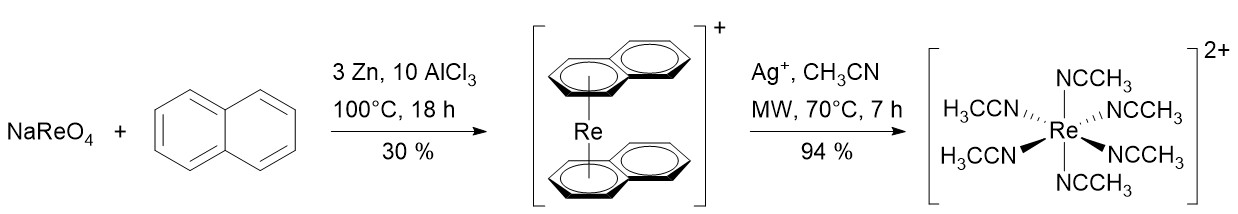
Fully solvated metal centers are useful precursors in coordination chemistry, as they solely consist of the metal core surrounded by one specific type of ligand, namely solvent molecules. The solvent molecules stabilize the metal center in its oxidation state, but are still easily exchanged which enables substitution reactions. Examples for such fully solvated precursors are the hexaqua complexes of the first row transition metals such as [Co(H2O)6]2+. Such hexaaqua complexes have so far not been found for Tc or Re, though there are some examples of fully solvated complexes with acetonitrile as ligand such as the dimeric [Re2(NCCH3)10]4+ or the monomeric [99Tc(NCCH3)6]2+.[1,2] Recently, we were able to synthesize the fully solvated and monomeric [Re(NCCH3)6]2+ by AgI mediated oxidation of [Re(η6-C10H8)2]+ in acetonitrile. The unexpectedly stable [Re(NCCH3)6]2+ was fully characterized and its potential as a ReII precursor complex was examined with various substitution reactions with phosphines and halides. Additionally, using DFT calculations we propose a possible mechanism for the formation of [Re(NCCH3)6]2+.[3]
[1] Stacey N. Bernstein, Kim R. Dunbar, Angew. Chem., 1992, 104, 1412-1414.
[2] F. Albert Cotton, Steven C. Haefner, Alfred P. Sattelberger, J. Am. Chem. Soc., 1996, 118, 5486-5487.
[3] Robin Bolliger, Giuseppe Meola, Henrik Braband, Olivier Blacque, Lukas Siebenmann, Qaisar Nadeem, Roger Alberto, Inorg. Chem., 2020, 59, 17600-17607.