Catalyst Repurposing Sequential Catalysis
The development of operationally simple multicatalytic systems by combining the advantages of both metal- and organocatalysis enables distinct transformations or reaction cascades otherwise inaccessible by single catalyst systems. This allows for unique possibilities for the formation of valuable organic frameworks. We herein describe an efficient and general catalyst repurposing strategy for the asymmetric synthesis of α-hydroxy-γ-butyrolactones. Starting from inexpensive and readily available starting materials our method is based on a sequential aldol addition/transfer hydrogenation/lactonization sequence by exploiting the inherent functionalities of prolineamides to serve as both an organocatalyst in an initial stereoselective aldol addition and as a ligand to promote a highly efficient transfer hydrogenation. This approach therefore represents an operationally simple, economic and efficient method for the preparation of the desired scaffolds. Furthermore, the developed catalyst repurposing sequential catalysis (CRSC) strategy was successfully applied for the selective preparation of key industrial intermediate (R)-pantolactone (vitamin B5 precursor), offering an attractive alternative to the currently established resolution strategy.[1]
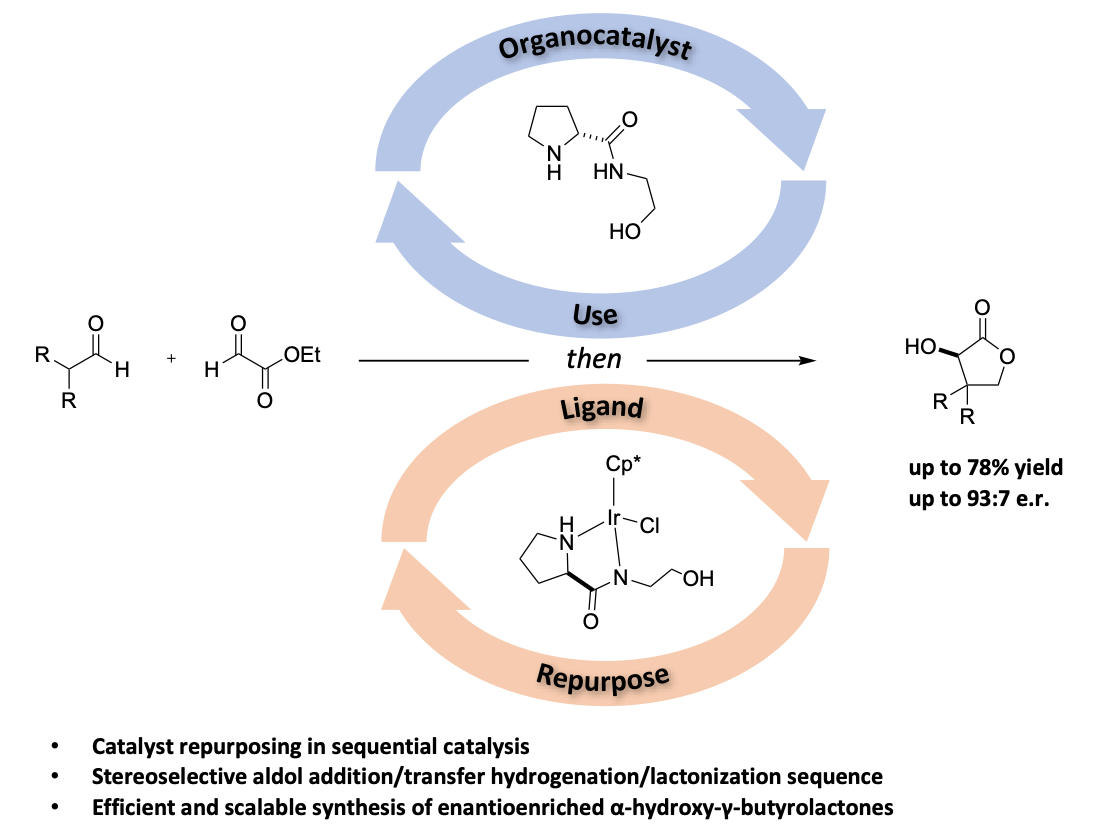
[1] F. Bourgeois, J. A. Medlock, W. Bonrath, C. Sparr, Org. Lett. 2020, 22, 110 – 115.