Disruption of mitochondrial redox homeostasis by enzymatic activation of a trialkylphosphine probe
Intracellular redox balance is crucial for cell function and is primarily regulated by the relative concentrations of glutathione (GSH) and its oxidized dimer (GSSG). Multiple physiological processes, ranging from cell signaling to protein folding depend on redox homeostasis. In eukaryotes, this homeostasis is controlled at the level of subcellular compartments and each organelle possesses its own redox environment.
Mitochondria fulfill multiple essential tasks in the cell that depend on redox modulation. Disruption of this homeostasis leads to pathologies such as insulin resistance and type II diabetes. Whereas the effects of oxidative stress in mitochondria have been thoroughly investigated, reductive stress has remained significantly underexplored. We envisioned that the GSH/GSSG ratio could be manipulated by direct reduction of the disulfide bond in GSSG to GSH using trialkylphosphine derivatives. We could achieve mitochondria targeting by taking advantage of the local enzymatic activity of nitroreductases, triggering the release of tributylphosphine and a fluorescent reporter from a masked precursor.
A series of live cells experiments including fluorescence imaging and biological assays showed that reductive stress is eventually transformed into oxidative stress through the production of O2–. It activates the ATF4-ATF3-CHOP cascade, which upregulates the CHAC1 gene. This response is mediated by glutathione, suggesting a link between reductive and oxidative stress.
In this work, we have demonstrated the importance of trialkylphosphines as chemical probes to modulate redox biology by developing strategies to tune their reactivity and subcellular targeting. These probes have a potential impact in the development of new therapies and enriching our understanding of subcellular compartmentalization of redox signaling.
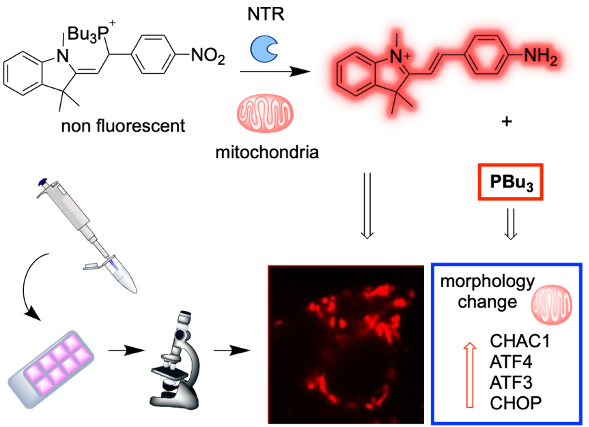
Figure. Mechanism of enzymatic activation of the probe by nitroreductases in mitochondria with simultaneous release of thributylphosphine and fluorescent reporter. Fluorescence microscopy shows localization in mitochondria. Transcriptomics analysis shows upregulation of genes from the integrated stress response.
J. Nguyen, A. Tirla, P. Rivera-Fuentes, Org. Biomol. Chem. 2021, 2681-2687.