Asymmetric, visible light-mediated radical sulfinyl-Smiles rearrangement to access all-carbon quaternary stereocentres
The development of novel strategies to disrupt symmetry at a molecular level is a longstanding pursuit within the organic chemistry community. Despite their importance in many biological active molecules, the asymmetric construction of all-carbon quaternary centers represents a major challenge for synthetic chemists. Acyclic systems are especially challenging due to the greater degrees of freedom, and steric congestion at the stereogenic center. Strategies involving both single and multiple C-C bond-forming events per chemical step have been developed, but sensitive reagents and careful temperature control are typically required.[1,2,3,4]
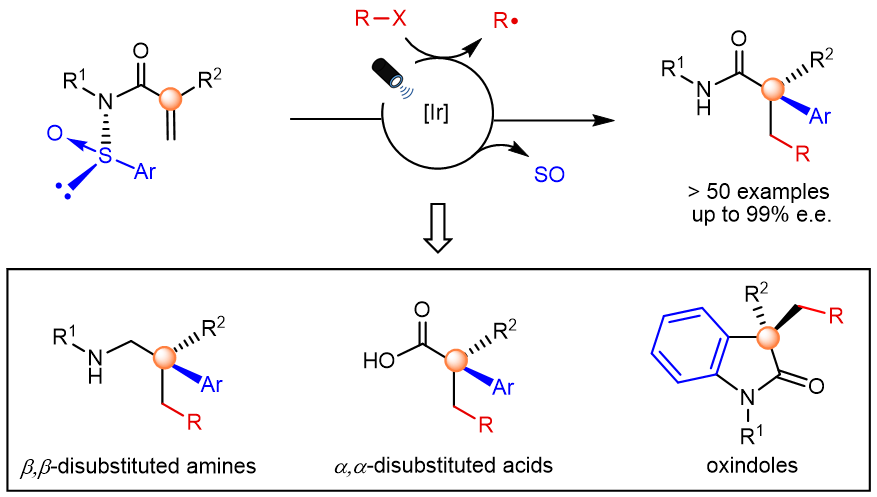
Here, we present the enantioselective synthesis of α-aryl-β-substituted amides bearing an all-carbon quaternary center.[5] The reaction proceeds via a radical cascade Smiles rearrangement triggered by photoredox catalysis where a sulfoxide group serves as a chiral auxiliary before being expelled as SO. The a-all-carbon substituted amides obtained in this process are prevalent in pharmaceuticals, agrochemicals and bioactive natural products. Further, they can be transformed, in a single additional step, into highly valuable chiral building blocks difficult to obtain by other methods.
[1] Jennifer A. Dabrowski, Matthew Villaume, Amir Hoveyda, Angew. Chem. Int. Ed. 2013, 52, 8156.
[2] Rauful Alam, Tobias Vollgraff, Lars Eriksson, Kalma ́ n J. Szabo, J. Am. Chem. Soc. 2015, 137, 11262.
[3] Ilan Marek, Yury Minko, Morgane Pasco, Tom Mejuch, Noga Gilboa, Helena Chechik, and Jaya P. Das, J. Am. Chem. Soc. 2014, 136, 2682.
[4] Michael Holmes, Khoa D. Nguyen, Leyah A. Schwartz, Tom Luong, Michael J. Krische, J. Am. Chem. Soc. 2017, 139, 8114.
[5] Cedric Hervieu, Mariia Kirillova, Tatiana Suárez, Marco Müller, Estibaliz Merino, Cristina Nevado, Nat. Chem. 2021, 13, 327.