Loop structures for strain sensing in elastomers
Mechanophores that exploit non-covalent interactions have gained recent interest due to their high reversibility and variable interaction strength.[1] Taking advantage of this concept, we developed polymers containing a rotaxane mechanophore that shows highly sensitive and reversible fluorescence switch-on behavior and a cyclophane mechanophore that displays ratiometric fluorescence color changes when subjected to uniaxial tensile deformation.[2] However, the synthesis of these motifs is complex and requires a large number of steps. Simple loop structures are an attractive alternative, since they are much more accessible, and related efforts in polymers gels have reported promising results.[3] We now report a non-covalent loop mechanophore with two covalently linked perylene diimide chromophores that was accessed in only three synthetic steps.[4] The incorporation of small amounts of this motif in poly(methyl acrylate) (PMA) and elastomeric polyurethanes (PU) with different hard segments was pursued to obtain solid polymer films that display the orange excimer emission of associated perylenes. In response to temperature or uniaxial tensile deformation, disruption of the dye aggregates occurs and an increase in green monomer emission is observed (Figure 1). An in-situ spectroscopic characterization corroborates that the intramolecular loop structure efficiently responds to the mechanical stimuli translating the applied strain into a defined optical response. Moreover, we demonstrate how complex stress relaxation processes and loading/unloading cycles can be monitored in samples that feature the loop mechanophores.
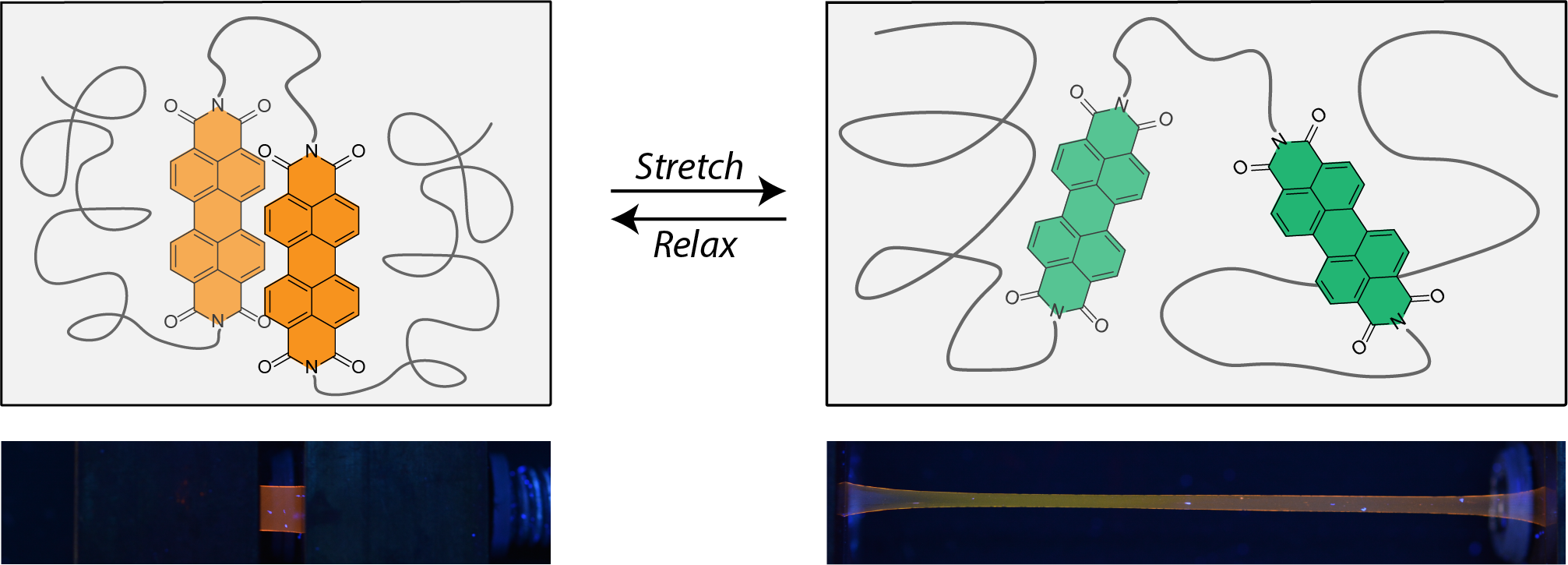
Figure 1 : Illustration of the working principle of a loop mechanophore (top) and photographs of polymer films bearing such loop mechanophores at 0 % and 1500 % strain (UV light illumination; λex = 365 nm).
[1] Hanna Traeger, Derek J. Kiebala, Christoph Weder, Stephen Schrettl, Macromolecular Rapid Communication 2021, 42, 2000573
[2] a)Yoshimitsu Sagara, Marc Karman, Ester Verde-Seto, Kazuya Matsuo, Yuna Kim, Nobuyuki Tamaoki, Christoph Weder, Journal of the American Chemical Society 2018, 140, 1584; b) Yoshimitsu Sagara, Hanna Traeger, Jie Li, Yuji Okado, Stephen Schrettl, Nobuyuki Tamaoki, Christoph Weder, Journal of the American Chemical Society. 2021, 143, 5519–5525
[3] Jian Chen, Adam W. Ziegler, Baoming Zhao, Wei Wan, Alexander D. Q. Li, Chemical Communications 2017, 53, 4993
[4] Hanna Traeger, Yoshimitsu Sagara, Derek J. Kiebala, Stephen Schrettl, Christoph Weder, Angewandte Chemie International Edition. 2021, https://doi.org/10.1002/anie.202105219