Tailoring a Sodium Amide (NaTMP) for Transition-Metal Free C-H Borylation of Arenes
Organoboron derivatives are key synthetic building blocks within everyday organic chemistry at both academic and industrial levels. One of their main applications is in Suzuki-Miyaura (SM) cross-couplings – a transformation which is estimated to represent over 65% of all C-C bond forming reactions performed.1 Organoboron compounds are frequently prepared by C-H borylation, wherein a relatively inert C-H bond is transformed into a more versatile C-BR2 bond.2 The majority of current methods require the use of transition-metal catalysts. Although metal-free examples do exist, substrate scope is often limited to electron-deficient heteroarenes.3
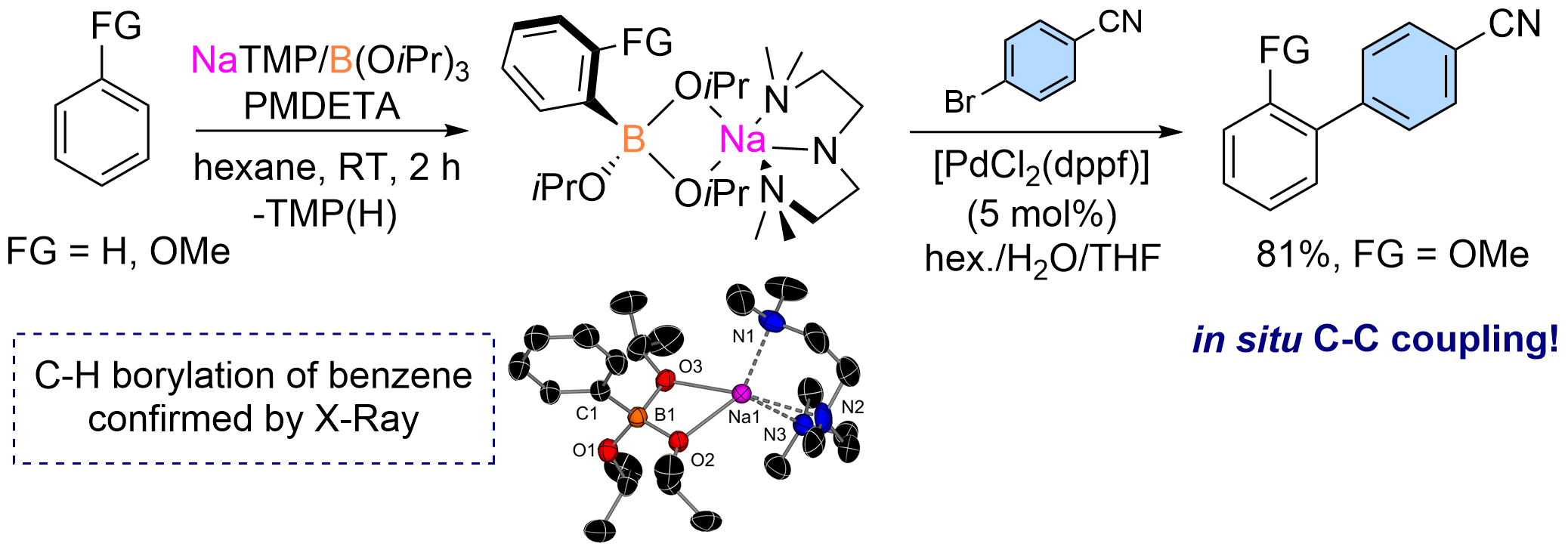
Combining the efforts of NaTMP (TMP = 2,2’,6,6’-tetramethylpiperidide) and commercially available B(OiPr)3, herein we introduce a new method for utilising a sodium amide as a metallating reagent in C-H borylation reactions. The reactivity of NaTMP is enhanced by the presence of tridentate Lewis donor PMDETA which induces its deaggregation, with B(OiPr)3 rapidly trapping and stabilising the metalated arene as shown in the scheme for benzene functionalisation. Isolation of key reaction intermediates provides important clues of the key roles of both Na and B in this borylation process. Showcasing the synthetic utility of this methodology, the newly formed sodium borate intermediates can then be used directly in SM cross-coupling reactions under moisture-tolerant conditions and in high yields.
[1] N. Schneider, D. M. Lowe, R. A. Sayle, M. A. Tarselli and G. A. Landrum, J. Med. Chem., 2016, 59, 4385-4402.
[2] I. A. I. Mkhalid, J. H. Barnard, T. B. Marder, J. M. Murphy and J. F. Hartwig, Chem. Rev., 2010, 110, 890-931.
[3] (a) S. A. Iqbal, J. Cid, R. J. Procter, M. Uzelac, K. Yuan and M. J. Ingleson, Angew. Chem. Int. Ed., 2019, 58, 15381-15385; (b) M. E. Grundy, K. Yuan, G. S. Nichol and M. J. Ingleson, Chem. Sci., 2021, doi:10.1039/D1SC01883C.